lib.mEoS.nC4¶
Fluid info¶
CAS Number: 106-97-8
Formula: CH3-(CH2)2-CH3
Synonym: R-600
Molecular weigth: 58.1222 g/mol
Tc: 425.1250 K
Pc: 3.7960 MPa
ρc: 228.0000 kg/m³
Tt: 134.8950 K
Tb: 272.6600 K
Acentric factor: 0.201
Dipole moment: 0.0500 Debye
Equation of state¶
Bücker, D., Wagner, W.; Reference Equations of State for the Thermodynamic Properties of Fluid Phase n-Butane and Isobutane. J. Phys. Chem. Ref. Data 35(2) (2006) 929-1019, http://dx.doi.org/10.1063/1.1901687
Younglove, B.A., Ely, J.F.; Thermophysical Properties of Fluids. II. Methane, Ethane, Propane, Isobutane, and Normal Butane. J. Phys. Chem. Ref. Data 16(4) (1987) 577-798, http://dx.doi.org/10.1063/1.555785
Kunz, O., Wagner, W.; The GERG-2008 Wide-Range Equation of State for Natural Gases and Other Mixtures: An Expansion of GERG-2004. J. Chem.Eng. Data 57(11) (2012) 3032-3091, http://dx.doi.org/10.1021/je300655b
Miyamoto, H., Watanabe, K.; A Thermodynamic Property Model for Fluid-Phase n-Butane. Int. J. Thermophys., 22(2) (2001) 459-475, http://dx.doi.org/10.1023/A:1010722814682
Span, R., Wagner, W.; Equations of state for technical applications. II. Results for nonpolar fluids.. Int. J. Thermophys. 24 (1) (2003) 41-109, http://dx.doi.org/10.1023/A:1022310214958
Polt, A., Platzer, B., Maurer, G.; Parameter der thermischen Zustandsgleichung von Bender fuer 14 mehratomige reine Stoffe. Chem. Technik 22(1992)6 , 216/224
Sun, L., Ely, J.F.; Universal equation of state for engineering application: Algorithm and application to non-polar and polar fluids. Fluid Phase Equilib., 222-223 (2004) 107-118, http://dx.doi.org/10.1016/j.fluid.2004.06.028
Viscosity¶
Herrmann, S., Vogel, E.; New Formulation for the Viscosity of n-Butane. J. Phys. Chem. Ref. Data 47(1) (2018) 013104, http://dx.doi.org/10.1063/1.5020802
Vogel, E., Küchenmeister, C., Bich, E.; Viscosity correlation for n-Butane in the Fluid Region. High Temp. - High Pressures 31(2) (1999) 173-186, http://dx.doi.org/10.1068/htrt154
Younglove, B.A., Ely, J.F.; Thermophysical Properties of Fluids. II. Methane, Ethane, Propane, Isobutane, and Normal Butane. J. Phys. Chem. Ref. Data 16(4) (1987) 577-798, http://dx.doi.org/10.1063/1.555785
Quiñones-Cisneros, S.E., Deiters, U.K.; Generalization of the Friction Theory for Viscosity Modeling. J. Phys. Chem. B, 110(25) (2006) 12820-12834, http://dx.doi.org/10.1021/jp0618577
Thermal Conductivity¶
Perkins, R.A, Ramires, M.L.V., Nieto de Castro, C.A., Cusco, L.; Measurement and Correlation of the Thermal Conductivity of Butane from 135 K to 600 K at Pressures to 70 MPa. J. Chem. Eng. Data 47(5) (2002) 1263-1271, http://dx.doi.org/10.1021/je0101202
Younglove, B.A., Ely, J.F.; Thermophysical Properties of Fluids. II. Methane, Ethane, Propane, Isobutane, and Normal Butane. J. Phys. Chem. Ref. Data 16(4) (1987) 577-798, http://dx.doi.org/10.1063/1.555785
Calculation example¶
Using the first option for equation of state, we can get this diagram plots with the liquid-gas saturation region:
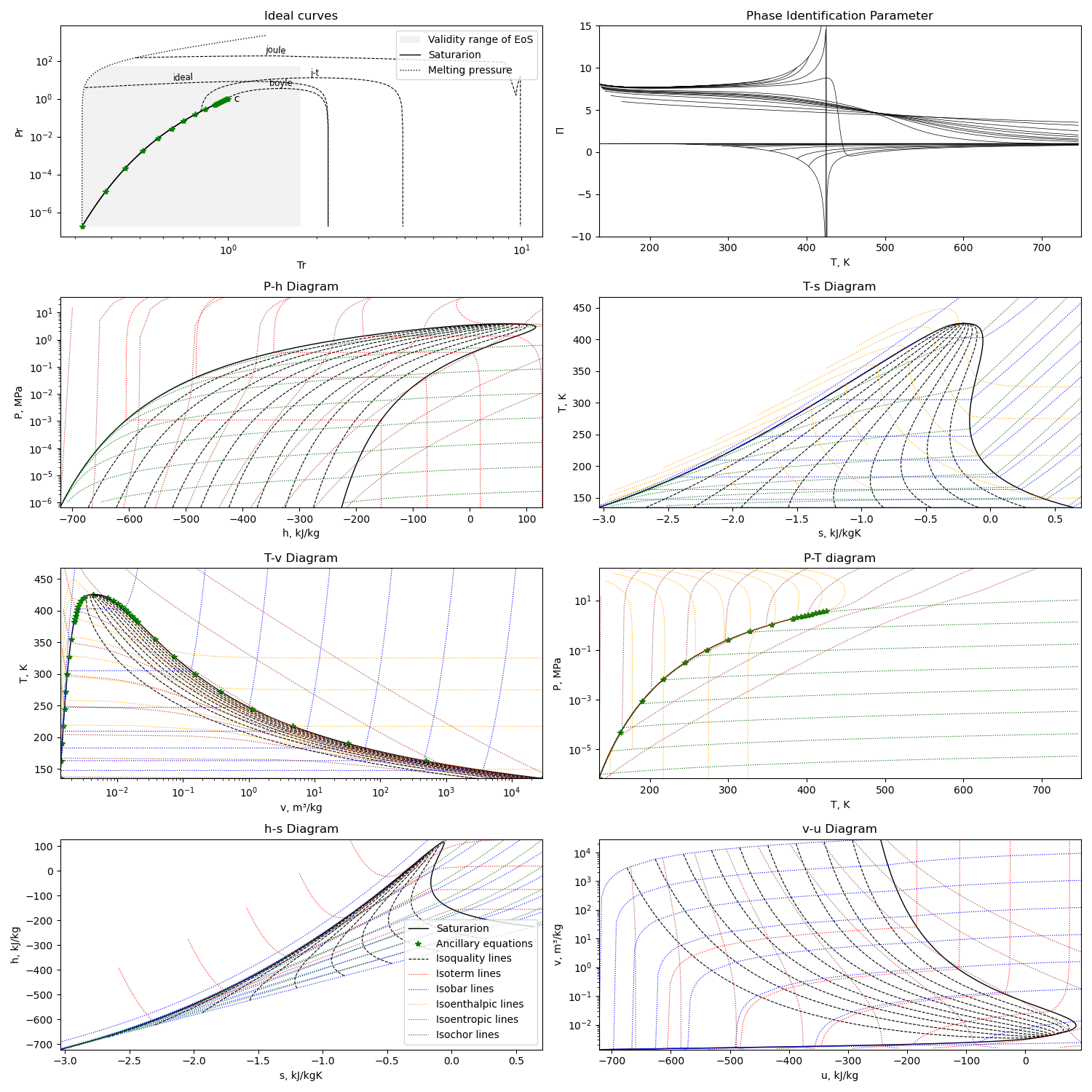
The diagram is generated with this module running with the compound name as parameter or edited in file
python3 plotMEoS.py nC4
