lib.mEoS.N2¶
Fluid info¶
CAS Number: 7727-37-9
Formula: N2
Synonym: R-728
Molecular weigth: 28.01348 g/mol
Tc: 126.1920 K
Pc: 3.3958 MPa
ρc: 313.3000 kg/m³
Tt: 63.1510 K
Tb: 77.3550 K
Acentric factor: 0.0372
Dipole moment: 0.0 Debye
Equation of state¶
Span, R., Lemmon, E.W., Jacobsen, R.T, Wagner, W., Yokozeki, A.; A Reference Equation of State for the Thermodynamic Properties of Nitrogen for Temperatures from 63.151 to 1000 K and Pressures to 2200 MPa. J. Phys. Chem. Ref. Data 29(6) (2000) 1361-1433, http://dx.doi.org/10.1063/1.1349047
Younglove, B.A.; Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data, 11(Suppl. 1) (1982)
Kunz, O., Wagner, W.; The GERG-2008 Wide-Range Equation of State for Natural Gases and Other Mixtures: An Expansion of GERG-2004. J. Chem.Eng. Data 57(11) (2012) 3032-3091, http://dx.doi.org/10.1021/je300655b
Jacobsen, R.T, Stewart, R.B., Jahangiri, M.; Thermodynamic Properties of Nitrogen from the Freezing Line to 2000K at Pressures to 1000MPa. J. Phys. Chem. Ref. Data, 15(2) (1986) 735-908, http://dx.doi.org/10.1007/BF00502385
Span, R., Wagner, W.; Equations of state for technical applications. II. Results for nonpolar fluids.. Int. J. Thermophys. 24 (1) (2003) 41-109, http://dx.doi.org/10.1023/A:1022310214958
Sun, L., Ely, J.F.; Universal equation of state for engineering application: Algorithm and application to non-polar and polar fluids. Fluid Phase Equilib., 222-223 (2004) 107-118, http://dx.doi.org/10.1016/j.fluid.2004.06.028
Viscosity¶
Lemmon, E.W., Jacobsen, R.T.; Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Int. J. Thermophys., 25(1) (2004) 21-69, http://dx.doi.org/10.1023/B:IJOT.0000022327.04529.f3
Younglove, B.A.; Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data, 11(Suppl. 1) (1982)
Stephan, K., Krauss, R., Laesecke, A.; Viscosity and Thermal Conductivity of Nitrogen for a Wide Range of Fluid States. J. Phys. Chem. Ref. Data 16(4) (1987) 993-1023, http://dx.doi.org/10.1063/1.555798
Thermal Conductivity¶
Lemmon, E.W., Jacobsen, R.T.; Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Int. J. Thermophys., 25(1) (2004) 21-69, http://dx.doi.org/10.1023/B:IJOT.0000022327.04529.f3
Younglove, B.A.; Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data, 11(Suppl. 1) (1982)
Stephan, K., Krauss, R., Laesecke, A.; Viscosity and Thermal Conductivity of Nitrogen for a Wide Range of Fluid States. J. Phys. Chem. Ref. Data 16(4) (1987) 993-1023, http://dx.doi.org/10.1063/1.555798
Calculation example¶
Using the first option for equation of state, we can get this diagram plots with the liquid-gas saturation region:
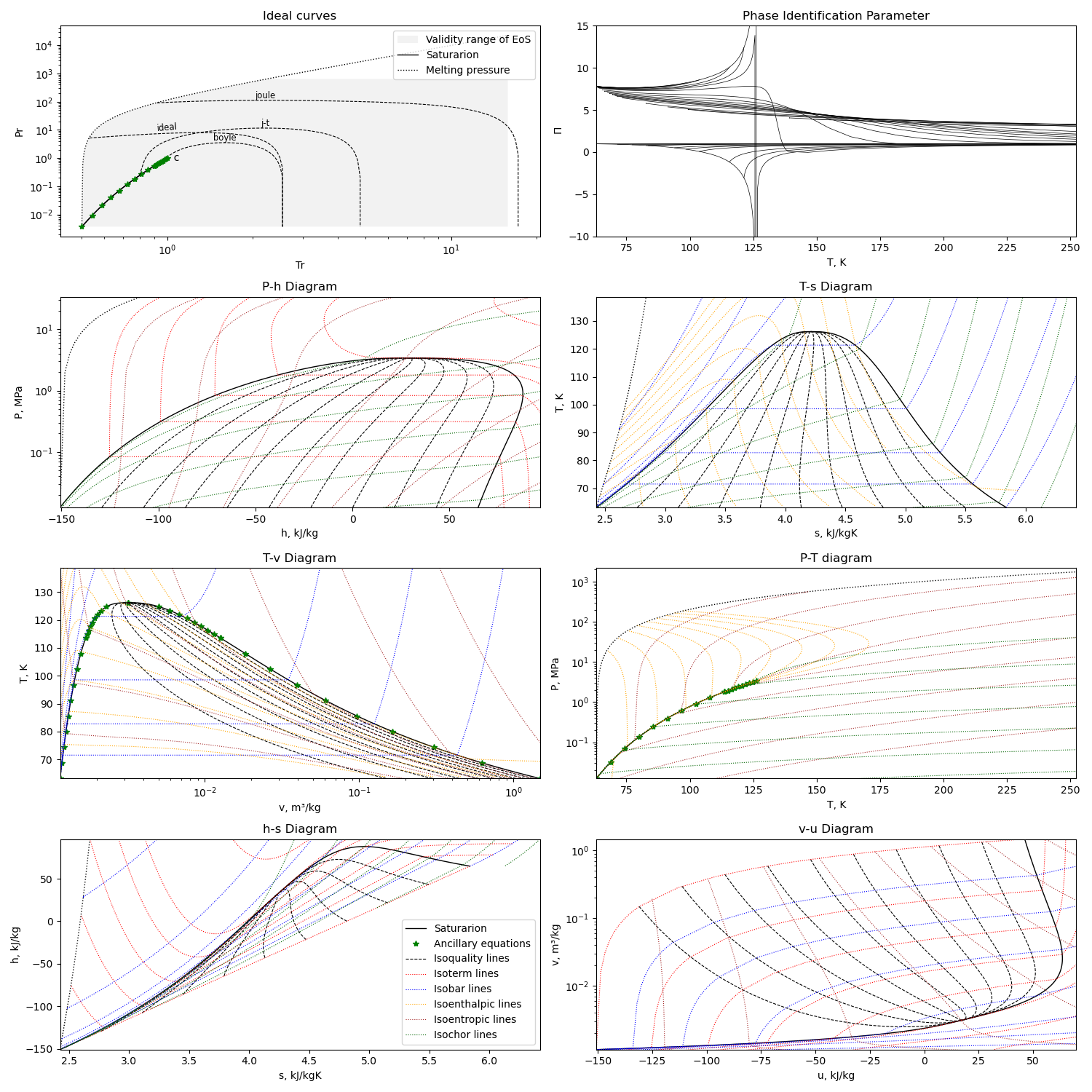
The diagram is generated with this module running with the compound name as parameter or edited in file
python3 plotMEoS.py N2
