lib.mEoS.C2¶
Fluid info¶
CAS Number: 74-84-0
Formula: CH3CH3
Synonym: R-170
Molecular weigth: 30.06904 g/mol
Tc: 305.3220 K
Pc: 4.8722 MPa
ρc: 206.1800 kg/m³
Tt: 90.3680 K
Tb: 184.5690 K
Acentric factor: 0.0995
Dipole moment: 0.0 Debye
Equation of state¶
Bücker, D., Wagner, W.; A Reference Equation of State for the Thermodynamic Properties of Ethane for Temperatures from the Melting Line to 675 K and Pressures up to 900 MPa. J. Phys. Chem. Ref. Data 35(1) (2006) 205-266, http://dx.doi.org/10.1063/1.1859286
Younglove, B.A., Ely, J.F.; Thermophysical Properties of Fluids. II. Methane, Ethane, Propane, Isobutane, and Normal Butane. J. Phys. Chem. Ref. Data 16(4) (1987) 577-798, http://dx.doi.org/10.1063/1.555785
Kunz, O., Wagner, W.; The GERG-2008 Wide-Range Equation of State for Natural Gases and Other Mixtures: An Expansion of GERG-2004. J. Chem.Eng. Data 57(11) (2012) 3032-3091, http://dx.doi.org/10.1021/je300655b
Friend, D.G., Ingham, H., Ely, J.F.; Thermophysical Properties of Ethane. J. Phys. Chem. Ref. Data 20(2) (1991) 275-347, http://dx.doi.org/10.1063/1.555881
Span, R., Wagner, W.; Equations of state for technical applications. II. Results for nonpolar fluids.. Int. J. Thermophys. 24 (1) (2003) 41-109, http://dx.doi.org/10.1023/A:1022310214958
Sun, L., Ely, J.F.; Universal equation of state for engineering application: Algorithm and application to non-polar and polar fluids. Fluid Phase Equilib., 222-223 (2004) 107-118, http://dx.doi.org/10.1016/j.fluid.2004.06.028
Viscosity¶
Herrmann, S., Hellmann, R., Vogel, E.; Update: Reference Correlation for the Viscosity of Ethane [J. Phys. Chem. Ref. Data 44, 043101 (2015)]. J. Phys. Chem. Ref. Data 44(4) (2015) 043101, http://dx.doi.org/10.1063/1.4930838
Vogel, E., Span, R., Herrmann, S.; Reference Correlation for the Viscosity of Ethane. J. Phys. Chem. Ref. Data 44(4) (2015) 043101, http://dx.doi.org/10.1063/1.4930838
Friend, D.G., Ingham, H., Ely, J.F.; Thermophysical Properties of Ethane. J. Phys. Chem. Ref. Data 20(2) (1991) 275-347, http://dx.doi.org/10.1063/1.555881
Younglove, B.A., Ely, J.F.; Thermophysical Properties of Fluids. II. Methane, Ethane, Propane, Isobutane, and Normal Butane. J. Phys. Chem. Ref. Data 16(4) (1987) 577-798, http://dx.doi.org/10.1063/1.555785
Quiñones-Cisneros, S.E., Deiters, U.K.; Generalization of the Friction Theory for Viscosity Modeling. J. Phys. Chem. B, 110(25) (2006) 12820-12834, http://dx.doi.org/10.1021/jp0618577
Thermal Conductivity¶
Friend, D.G., Ingham, H., Ely, J.F.; Thermophysical Properties of Ethane. J. Phys. Chem. Ref. Data 20(2) (1991) 275-347, http://dx.doi.org/10.1063/1.555881
Younglove, B.A., Ely, J.F.; Thermophysical Properties of Fluids. II. Methane, Ethane, Propane, Isobutane, and Normal Butane. J. Phys. Chem. Ref. Data 16(4) (1987) 577-798, http://dx.doi.org/10.1063/1.555785
Calculation example¶
Using the first option for equation of state, we can get this diagram plots with the liquid-gas saturation region:
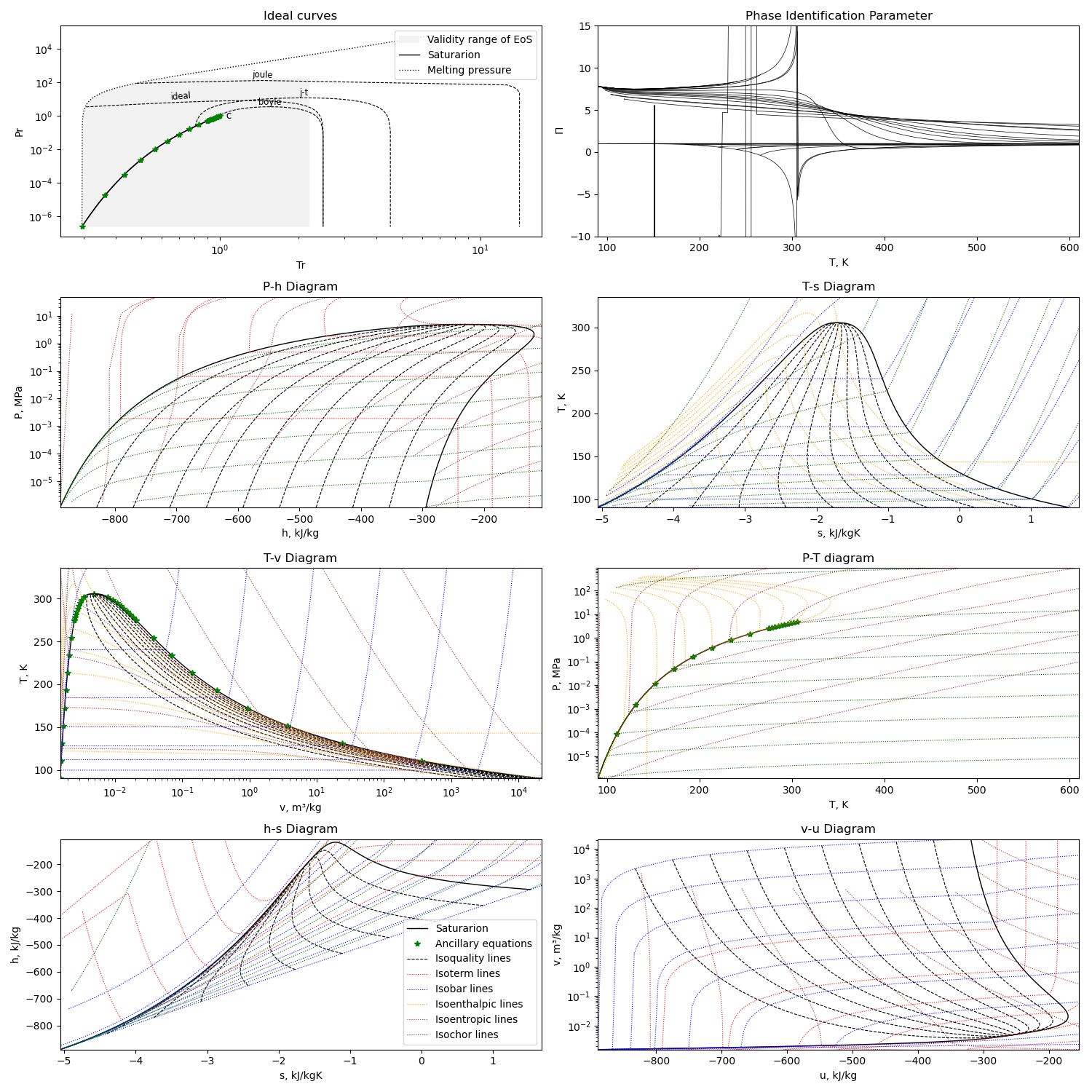
The diagram is generated with this module running with the compound name as parameter or edited in file
python3 plotMEoS.py C2
