lib.mEoS.Ar¶
Fluid info¶
CAS Number: 7440-37-1
Formula: Ar
Synonym: R-740
Molecular weigth: 39.948 g/mol
Tc: 150.6870 K
Pc: 4.8630 MPa
ρc: 535.6000 kg/m³
Tt: 83.8058 K
Tb: 87.3020 K
Acentric factor: -0.00219
Dipole moment: 0.0 Debye
Equation of state¶
Tegeler, Ch., Span, R., Wagner, W.; A New Equation of State for Argon Covering the Fluid Region for Temperatures From the Melting Line to 700 K at Pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 28, 779 (1999), http://dx.doi.org/10.1063/1.556037
Younglove, B.A.; Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data, 11(Suppl. 1) (1982)
Kunz, O., Wagner, W.; The GERG-2008 Wide-Range Equation of State for Natural Gases and Other Mixtures: An Expansion of GERG-2004. J. Chem.Eng. Data 57(11) (2012) 3032-3091, http://dx.doi.org/10.1021/je300655b
Stewart, R.B., Jacobsen, R.T.; Thermodynamic Properties of Argon from the Triple Point to 1200 K at Pressures to 1000 MPa. J. Phys. Chem. Ref. Data, 18(2):639-798, 1989, http://dx.doi.org/10.1063/1.555829
Span, R., Wagner, W.; Equations of state for technical applications. II. Results for nonpolar fluids.. Int. J. Thermophys. 24 (1) (2003) 41-109, http://dx.doi.org/10.1023/A:1022310214958
Viscosity¶
Lemmon, E.W., Jacobsen, R.T.; Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Int. J. Thermophys., 25(1) (2004) 21-69, http://dx.doi.org/10.1023/B:IJOT.0000022327.04529.f3
Younglove, B.A., Hanley, H.J.M.; The Viscosity and Thermal Conductivity Coefficients of Gaseous and Liquid Argon. J. Phys. Chem. Ref. Data 15(4) (1986) 1323-1337, http://dx.doi.org/10.1063/1.555765
Younglove, B.A.; Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data, 11(Suppl. 1) (1982)
Thermal Conductivity¶
Lemmon, E.W., Jacobsen, R.T.; Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Int. J. Thermophys., 25(1) (2004) 21-69, http://dx.doi.org/10.1023/B:IJOT.0000022327.04529.f3
Younglove, B.A.; Thermophysical Properties of Fluids. I. Argon, Ethylene, Parahydrogen, Nitrogen, Nitrogen Trifluoride, and Oxygen. J. Phys. Chem. Ref. Data, 11(Suppl. 1) (1982)
Perkins, R.A., Friend, D.G., Roder, H.M., Nieto de Castro, C.A.; Thermal Conductivity Surface of Argon: A Fresh Analysis. Int. J. Thermophys., 12(6) (1991) 965-984, http://dx.doi.org/10.1007/BF00503513
Younglove, B.A., Hanley, H.J.M.; The Viscosity and Thermal Conductivity Coefficients of Gaseous and Liquid Argon. J. Phys. Chem. Ref. Data 15(4) (1986) 1323-1337, http://dx.doi.org/10.1063/1.555765
Calculation example¶
Using the first option for equation of state, we can get this diagram plots with the liquid-gas saturation region:
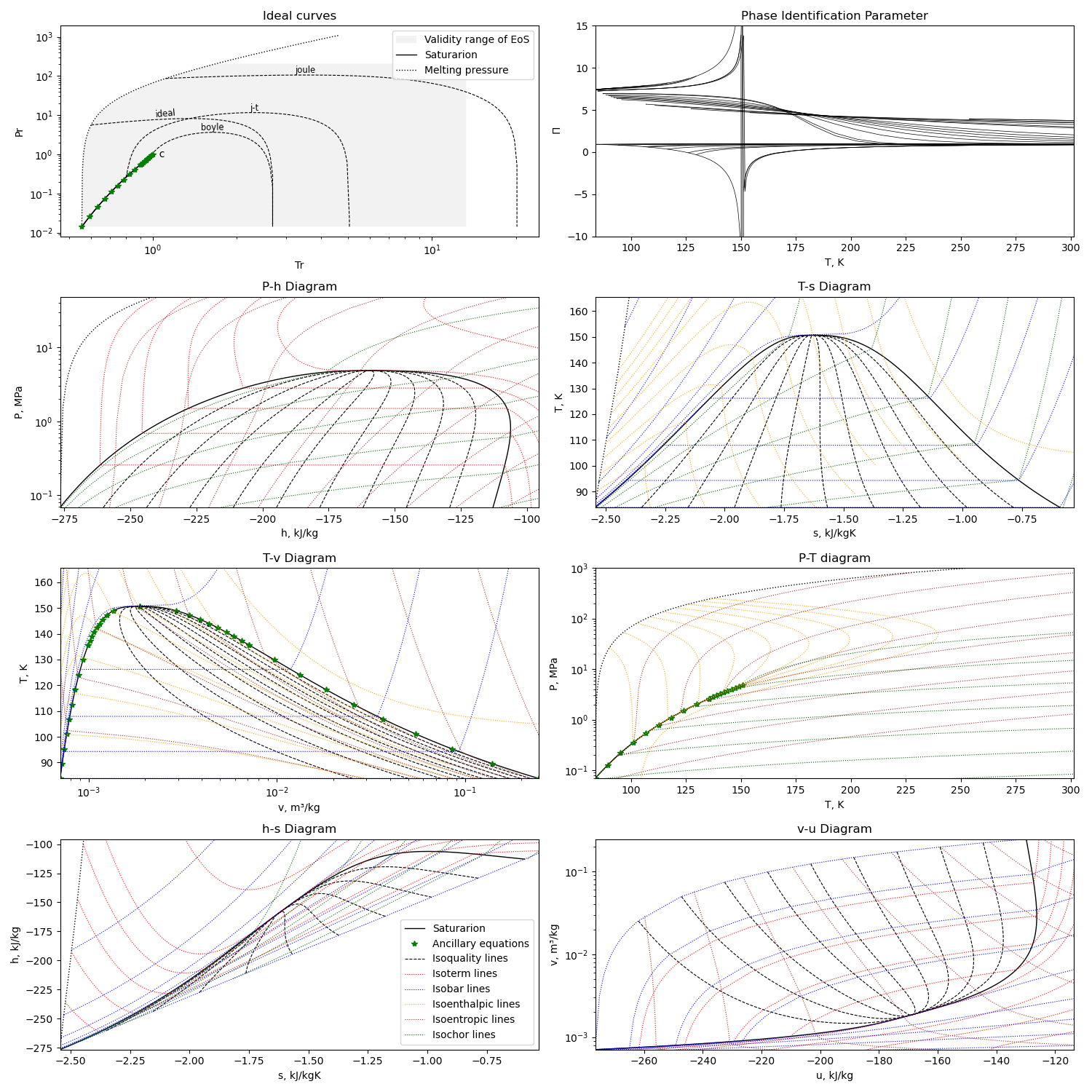
The diagram is generated with this module running with the compound name as parameter or edited in file
python3 plotMEoS.py Ar
